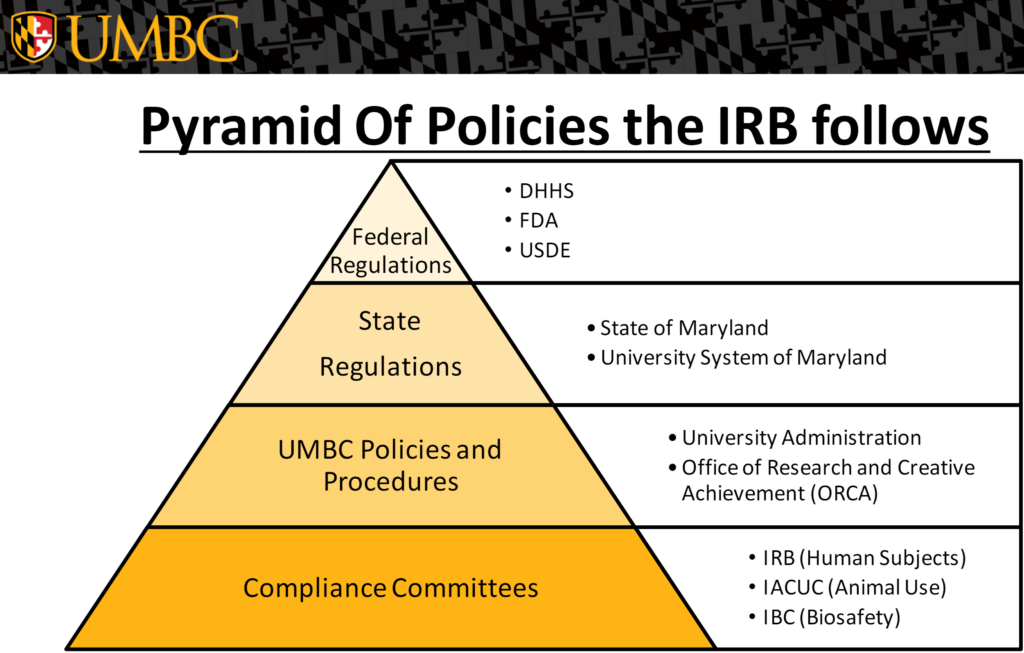
UMBC’s Department of Human Research Protections and Integrity (HRPI) supports the Institutional Review Board (IRB) and its Federalwide Assurance, which protects the rights and safety of human subjects participating in research. The IRB requires that all investigators who are affiliated with UMBC and who are engaged in research, regardless of funding, comply with both UMBC procedures and federal regulations regarding the protection of human subjects in protocol activities.
IRB Announcements
- Student PIs of IRB protocol(s) may be contacted to submit a closure report before they graduate. Student PIs of non-exempt IRB protocols may be asked to participate in a Post Approval Monitoring review prior to their graduation date.
- Beginning December 1, 2024, submit new Scholarship of Teaching & Learning (SoTL) IRB protocol applications directly through UMBC’s IRB Kuali portal. Existing SoTL IRB protocols will continue to be managed by University of Maryland, College Park through July 14, 2026.
- UMBC research activities involving minors require fingerprinting & background checks. Kuali IRB protocol applications guide PIs and research personnel in completing the required fingerprinting and background check through Talent Acquisition, before IRB approval. PIs who plan to interact with child participants will be asked to submit this supplementary form to HR.
IRB Quarterly Newsletters
Do I Need an IRB Protocol for my Research?
Overview of the UMBC IRB Process
The UMBC Institutional Review Board (IRB) has responsibility of providing oversights of research activities involving human subjects and ensuring that ethical standards for safeguards and protection of participants.
Research with human subjects, regardless of funding, conducted under the auspices of UMBC, is reviewed approved by the IRB in compliance with federal regulations, its Federalwide Assurance and institutional policies and procedures. The University of Maryland, Baltimore County has a Federalwide Assurance (IRB registration number: IORG0000202 / FWA number: 00000069 approved through 06/16/2026) from the Office for Human Research Protections. Protocols describing research with human subjects must be submitted to and approved by the IRB before research use can begin.
Additionally, all personnel involved in protocol involving humans must complete required training prior to conducting the research.
Briefly, the protocol review process entails:
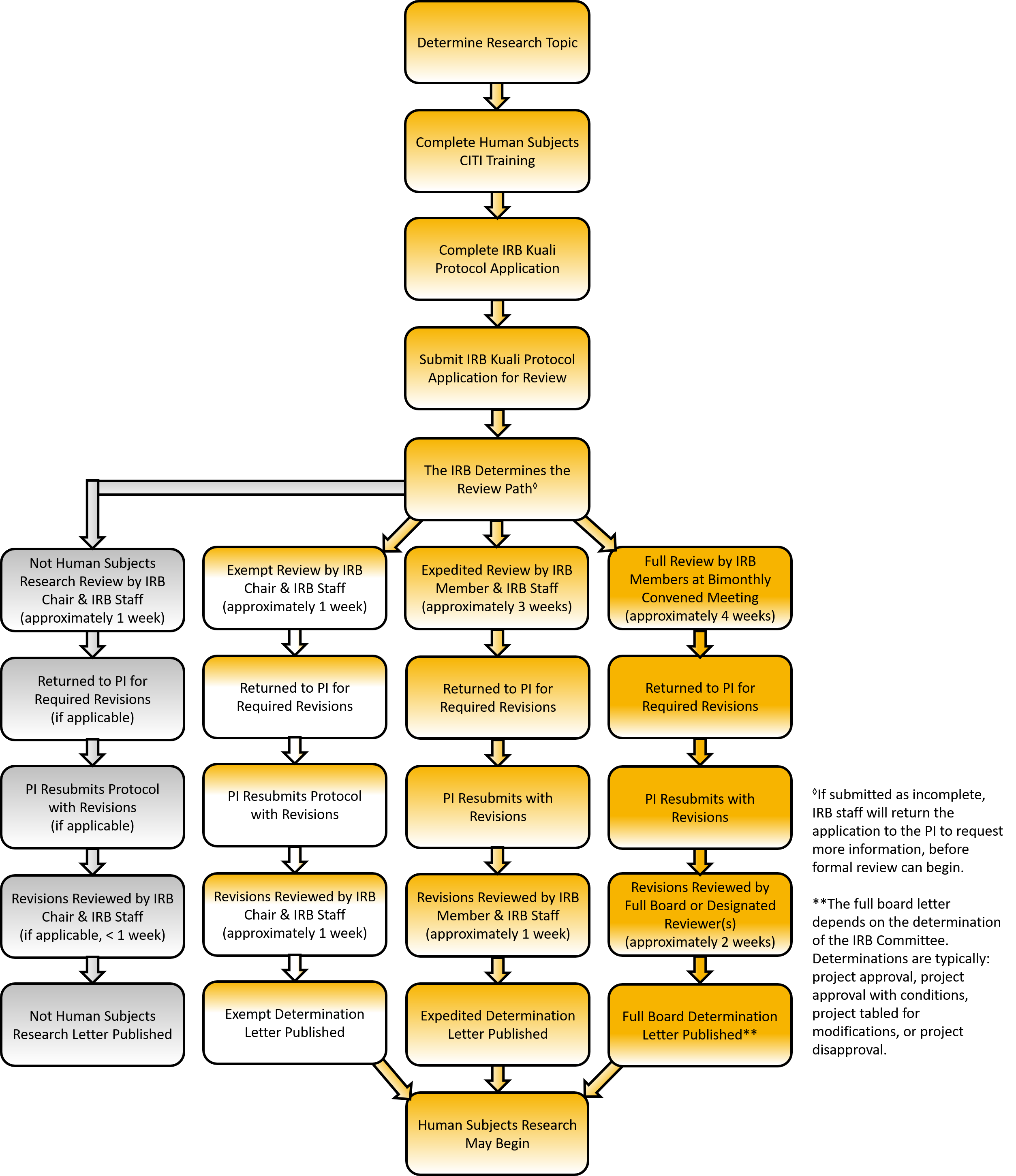
1) Completion and submission of a Kuali IRB protocol.
UMBC IRB Protocol Creation and Submission Process
This information will assist investigators in the planning, development and submission of human participant use protocols for review and approval by the IRB. Review types are described below. The below submission type provides a link to the Kuali Protocols Portal as well as the IRB Protocol User Guide. The Guide has a searchable table of contents to the specific sections to assist in creating a protocol.
Kuali is a cloud-based electronic research administration tool. Use Kuali for IRB protocol submissions.
Link to Kuali Protocols – umbc.kuali.co/protocols/
Link to Kuali Information – Kuali At UMBC
Link to Kuali Protocol Training Guides
Who Can Be an IRB Principal Investigator and Who Are Study Personnel?
Principal Investigator
A Principal Investigator (PI) is someone who is involved in conducting human subjects research studies and has the primary responsibility for the design and implementation of research involving human subjects. The IRB considers persons eligible as a PI who has a faculty appointment (full/part time) the department where human subjects research activities are conducted. This allows persons to create, submit and manage protocols in the Kuali Protocols system. The PI must attest in an IRB application that they, as well as all research staff involved in the study, are aware of and understand their individual and collective responsibilities for human subjects research related tasks including obtaining informed consent, creating and following procedures for safeguards and protections of human research participant well-being, personnel training, etc.
Undergraduate students and Masters or Doctoral Candidates may serve as a PI, but a faculty member must co-sign an application and serve as a “research advisor” for the protocol. The student’s as well as the research advisor’s human subjects research expertise and training is evaluated by the IRB. Student investigators do have the ability to create a Kuali IRB protocol and submit to the IRB for review and approval. Faculty advisors must review and “sign off” the protocol before the IRB performs its review. The process to follow is explained in the IRB Protocol User Guide.
The IRB expects the students and faculty advisors to be knowledgeable about human research regulations , IRB procedures as well as complete required CITI human subjects training courses. It is expected by the IRB that research advisors devote time and effort in evaluating a student’s proposed project, supervise the student in the conduct of the research, assist the student in solving issues and be the resource to the student in promptly reporting incidents to the IRB.
Study Personnel
Kuali pull personnel data from HR; this allows for ease of adding current personnel to a protocol. Anyone listed on the study can prepare changes to the study; however, only the PI or can submit the change.
Adding Study Team Members
- All study team members must have completed the required IRB training in CITI before creating a role in Kuali. The IRB will verify this training during review
- Select which Researcher Role personnel will follow.
- Describe, where applicable, in the data access and consent document/processes sections how personnel are involved
Changing Study Team Members
- Changes to study team members must have completed the required IRB training in CITI before creating a role in Kuali. The IRB will verify this training during review Select which Researcher Role personnel will follow.
- Update, where applicable, in the data access and consent document/processes sections how personnel are involved
Add study team members from external institutions or sites
When a PI is collaborating with research personnel from external (outside) organizations and institutions, those personnel cannot be added directly into Kuali. One option to do so is to request a Sponsored Account that provides temporary UMBC identities to access to Kuali Protocols. Faculty or staff member from the department performing research may request access from DoIT. This person will act as the affiliation sponsor for a period up to one year, with the option of extending the affiliate access.
Include in the protocol description the purpose for requesting access to Protocols and describe what roles and responsibilities they will have for the term of the affiliation with the human subjects research. Choose in the data access and consent document/processes sections how personnel are involved. Include evidence of human subjects research training in the protocol.
If an affiliation is not required, only provide a description in the protocol narrative on what responsibilities this person will perform in the research and if data access and consent document/processes are required.
Training
The IRB requires that all identified research personnel, including faculty, graduate students and undergraduate students complete the on-line education module. Training must be completed before submitting an application. Please review the requirements for human subjects use training.
Humans Subjects Research Protocol Submission Types
Principal investigators may make the initial determination about the type of review appropriate to the project for submission via Kuali IRB. However, final review determination rests with the IRB. If another type of review is more appropriate, the ORPC notifies the investigator to re-submit via Kuali IRB.
IRB Application and Pre-Review Consultation
The ORPC staff is available to answer any questions regarding the Kuali IRB submission process and IRB review of research studies. We also provide pre-review consultation to discuss all aspects of IRB review, from preparing the Kuali IRB submission to completion of the study. Questions or comments? Send them to IRB@umbc.edu.
When will a Principal Investigator hear from the IRB about study approval?
The length of time a study will take from submission to receiving an approval notification received depends on the type or level of review stated below. The ORPC staff, working in concert with the IRB, will make every effort to work with investigators to process protocol submissions promptly. If additional changes are needed, it can take longer depending on reviewer questions or protocol load.
Would my proposed project require IRB review?
That depends. There are examples of what “could” be considered Not Human Subjects Research. Contact the ORPC with information and your questions before starting the project.
Kuali IRB for Protocols must be used for all applications submitted for review. Please select the applicable review type and follow the instructions for submission procedures.
2) Review of a submitted protocol via the Exempt Review or Expedited Review processes or for full committee consideration at a scheduled meeting.
Exempt Review
The IRB Chair screens studies for exemption determination. Such research must fall within one of the federally designated exempt review categories and be no more than “minimal risk” If the study is not found exempt, it will need to go through expedited or full-committee review. This link describes the exempt application and review process. Exempt determinations take approximately two weeks.
Expedited Review
Members of the IRB review expedited review protocols to determine “minimal risk” to participants and fit into an expedited review category of research. This link describes the expedited application and review process. Expedited reviews take approximately four weeks.
Full-Committee Review
Protocols greater than “minimal risk” must be reviewed and discussed at a formally convened meeting of the IRB. Full-committee review protocols must be submitted no later than thirty (30) days before the scheduled meeting. This link describes the full board application and review process. The current committee meeting schedule is:
October 6, 2025
December 1, 2025
February 2, 2026
April 6, 2026
June 1, 2026
Classroom Projects
The IRB has developed guidelines to advise faculty instructors on what are acceptable topics, the use of the consent process, and faculty responsibilities for human subjects research class projects.
Student Research Using Data from a Faculty Advisor’s Protocol
Student researchers may wish to carry out research projects or studies that involve using data collected on a faculty advisor’s IRB approved protocol. Please follow this process to obtain either exempt or expedited IRB approval.
Secondary Data Use Research
Investigators who plan to use, study, or analyze restricted or identifiable private information for data for secondary research purposes will follow these steps for data use agreement procedures and to obtain IRB approval. On the other hand, research with publicly available, de-identified sources of data or if information can no longer be connected to the identity of the subjects, follow these steps.
Planning Phase Administrative Request
When an investigator has plans to perform human subjects research in the future that lead to the submission of grant applications, submit this planning phase administrative request
IRB Reliance Agreements (formerly called IAAs)
Involving external research collaborators on an IRB activity? Consider the potential of a reliance agreement
Not Human Subjects Research
Human subjects review depends on how regulatory requirements and IRB processes apply. Review the IRB’s guidance here.
3) Review of planning and development activities that lead to the submission of grant applications where human subjects use are planned in future but have not been finalized, or when a PI received a “just-in-time” notice from a granting agency and is requesting documentation of IRB approval.
4) Post approval research activities including amendments to research, reporting of deviations, non-compliance and adverse incidents and protocol closures.
A major investigator responsibility is to be compliant all IRB policies, decisions, conditions, and requirements. This means ensuring that the research is implemented as specified in the approved IRB protocol. Click on the below topics to review what is required after the IRB provides approval.
5) The involvement of external research collaborators using an IRB reliance agreement (IAA) process or directly recruiting participants from the UMBC community with that institution’s current IRB approval.
Policies, Procedures, & Guidance
Consent & Assent Guidance and Templates
The goal of the consent process is to facilitate a prospective participant’s or legally authorized representative’s understanding of the reasons why an individual might or might not want to participate in a research study. The responsibility for the investigator in the consent process is to:
- Provide more information when requested by subjects
- Make sufficient time and opportunity to discuss the research
- Answer questions to improve a subject’s understanding
Click Here for Additional Consent Information and Template Documents
IRB Charter & Board Member Manual
IRB Standard Operating Procedures (SOPs)
General Policies
IRB Board Membership Policies
Protocol Preparation & Documents
Protocol Review Process
- 401a Non-Human Subjects Research
- 401b Research Activities Exempt from IRB Review
- 402 Expedited Review
- 403 Initial Review – Criteria for IRB Approval
- 404 Continuing Review
- 405 Review of Amendments to Research Studies
- 406 Final Project Reports
- 407 IRB Actions to Approve or Disapprove Research
- 409 Alternative IRB Review Arrangements and Agreements (coming soon)
- 410 Appeal of IRB Decisions
- 411 IRB Protocol Renewal Period
Additional Participant Protections – Coming Soon!
IRB SOPs are in continuous development at this time. Please check back later and thank you for your patience!
Post-Approval Monitoring (PAM) Checklist
Required Training for Human Subject Researchers
All investigators, advisors and research team personnel (staff, students, and volunteers) who participate in the design and/or the conduct of human subjects research (including exempt research) must be appropriately trained in the protection of human subjects. Note that external evaluators, consultants or transcriptionist who are involved research, but will not have any contact with human participants or identifiable data, are not required to take this training.
Research personnel must complete the below required modules before the IRB provides approval. Personnel may also choose to supplemental coursework in each module specific to research project needs. Training module descriptions are available here. Follow instructions below. UMBC may accept evidence of training (e.g. certificate of completion) from another institution (see below). Upload Please forward these documents to compliance@umbc.edu for review.
CITI also offers Responsible Conduct of Research (RCR) Courses. While useful to inform on the aspects of RCR, these courses do not provide the content the IRB requires to fully inform investigators of the ethics and procedures of conducting human subjects research. These RCR modules cannot be substituted for the below training courses but are required for NSF, NIH, USDA funded research and some UMBC graduate Programs. Please be sure IRB training modules are only listed in the protocol application.
Questions or issues regarding training? Click this link: Research compliance feedback and reporting research concerns.
CITI Program Human Subjects Use Training
IMPORTANT NOTE: ALL HUMAN SUBJECTS USE TRAINING MUST BE REPEATED EVERY 5 YEARS.
First step:
- Using your UMBC login credentials, click on the University of Maryland, Baltimore County Single Sign On (SSO) login link on the CITI Program’s website.
- Persons with a current CITI login will be directed to their current CITI course list.
- If you do not have a current CITI login, you will see this screen. Click on the appropriate link and follow the below instructions
- Click on Register link in top right.
- Set up an account for yourself – in “Select Your Organization Affiliation” section, make sure to select “University of Maryland, Baltimore County” by typing it in box. Then follow the rest of the instructions to create your account.
- When asked “Which courses do you plan to take”…
- Scroll to “Question #1” and under “Human Subjects Courses” choose one of the following:
Students conducting less than minimal risk research – Appropriate for Undergraduate Students working on classroom research. All Undergraduate students working on independent research should take the Social/Behavioral course.
Social/Behavioral Research Course – Appropriate for All Students, Faculty and Staff
Research with pre-existing data, records data or laboratory specimens – Appropriate for researchers with no direct contact with Human subjects and/or Secondary Data Use
GCP – Social and Behavioral Research Best Practices for Clinical Research – Appropriate for researchers conducting research in a clinical setting
GCP for Biomedical Research – Appropriate for researchers working with FDA regulated drug, device or biologic studies.
- Scroll to “Question #7” and under “Information Privacy and Security” choose one of the following:
Health Information Privacy – Required for those working with HIPAA protected Data
Information Security Course – Suggested for all UMBC Researchers
Family Educational Rights and Privacy Act (FERPA) – Suggested for those working with data that is protected under FERPA
- Once courses selected, click “Submit”
- Then complete the courses
Your CITI training record is automatically fed into the Kuali IRB protocol record.
Once the initial training period has ended, CITI will provide instruction to renew training modules with a refresher course.
Non-UMBC affiliated users involved in human subjects research
Key research team members (e.g., co-principal investigators) from other institutions who are “engaged” in a UMBC IRB approved protocols must have a record of current human subjects protection training and recorded in the Kuali IRB protocol record. The IRB prefers members of the research team complete appropriate CITI training modules. Persons who have completed CITI training at another institution should affiliate their CITI account with UMBC. After affiliating with UMBC, CITI will ask you to complete any modules required by UMBC that may not have been required by the current research team member’s institution. Review CITI’s guidance on how to add/change your affiliated institution or transfer completions for more information.
The IRB will also accept completion of the Office for Human Research Protections Human Research Protection Training module for non-UMBC affiliated investigators who participate in UMBC IRB approved protocols. UMBC PIs are required to upload evidence of training completion in the protocol.
Additional Training Opportunities
UMBC’s Office of Research Protections and Compliance provides a wide variety of training. ORPC has presented to departments, labs and administrative groups on campus. We would be more than happy to meet with you or your unit. Please feel free to contact us at IRB@umbc.edu.
Special Topics in Human Subjects Research & IRB Protocols
These are areas the IRB has questions about from time to time. Investigators whose protocols will involve research in these areas will find the information under each area useful.
ABA Student Investigator Research Approval
Advertisements and recruitment materials
Assessing Decisional Capacity
Collaborative Research
Community-Based Participatory Research
Consent for Individuals With Cognitive Impairments
Disclosure of Financial Conflicts of Interest
Evaluating Risks
Focus Groups
Guidelines for Reporting Sensitive Information
HIPAA
Human Subjects and the General Data Protection Regulations (GDPR)
Human Tissues and Samples
International Research (scroll to center of this page)Online or Internet Research
MTurk Uses in Human Subjects Research
Participant Screening
Participant Compensation
Protocol Files
Recruitment from Outside of Campus
Research with Children
Risks Associated with Human Subjects Research
Student Research Using Data from a Faculty Advisor’s Protocol
UMBC Security Requirements for Protecting Sensitive Research Data
Use of Publicly available, De-Identified Sources of Data
Use of Restricted Data for Secondary Research Purposes Involving Identifiable Coded or Private Information
Using Interpreters in a Study
Using Transcriptionists for Recorded Interviews
IRB for the Scholarship of Teaching and Learning (SoTL)
SoTL research IRB protocols approved prior to December 1, 2024 will continue to reside with the IRB office of UMD College Park. Amendments and other communication regarding these existing SoTL protocols may continue to be directed to sotlresearch@umd.edu.
- USM Press Release
- Process Overview
- Description of Study Types under USM SoTL IRB
- Process to Join and Submit SoTL IRB Applications
- Amendment (SoTL IRB Application) Form
- Contact for Questions and Amendment (SoTL IRB Application) Submission: sotlresearch@umd.edu
Information for Prospective or Current Human Research Participants
Interested in serving on the IRB committee?
Kuali is a cloud-based electronic research administration tool. Use Kuali for IRB protocol submissions.
Link to Kuali Production – umbc.kuali.co
Link to Kuali Information – Kuali At UMBC
Link to Kuali Protocol Training Guides
Have a research compliance concern? See Research compliance feedback and reporting research concerns