Research demonstrates impact of collaboration across fields.
An interdisciplinary UMBC research team has combined developmental biology and mathematics research methods to reveal dramatic new insights on egg development in the latest issue of Nature Communications.
Michelle Starz-Gaiano, assistant professor of biological sciences, and Bradford Peercy, associate professor of mathematics and statistics, worked with Meyerhoff Graduate FellowLathiena Manning ’15 Ph.D., biological sciences, and Anne Marie Weideman ‘14, mathematics, a current master’s student and alumnus of the NSF-funded interdisciplinary training for Undergraduates in Biological and Mathematical Sciences (UBM) program.
As an animal develops, cells are "fated" to take on different functions, and once fated they go through migration, changes in shape, cell division, and growth to build that organism. Scientists have long sought to fully understand the intricacies of these processes, as errors can have serious consequences for the organism. For example, cell fate malfunctions can play a role in the development of cancers and cleft palate is caused by cells that are fated correctly but don't migrate correctly.
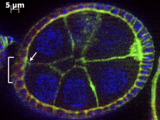
This new research in Nature Communications explores cell fates due to their spatial arrangements, specifically fruit fly ovarian cells that become motile if they get the right chemical signals. Previous science suggested that the closer a cell is to the secreted signal, the higher concentration of signal it would get, and the more likely it would be to become activated and turn into a motile cell. However, this isn't always the case.
Why don't all of the nearby cells respond as theory would predict? To find the answer, the researchers zoomed in for a closer look at the cells and found an important factor not previously examined.
This new paper suggests that the three-dimensional arrangement of cells in relation to one another impacts their response to signals. If you look closely, you can see tiny gaps around the cells, which don't fit completely flush against each other. If there is a gap, a cell is not getting as much activation.
A mathematical model outlined in the paper demonstrates that as that gap fills up with the signal, it shifts from acting as a sink that holds the signal into being a source of signal, which will flow from it. This means that there is a dynamic aspect of cell contours. If you change the landscape of underlying cells, you change activation patterns. Strikingly, the model predicted all of the activation patterns observed in the experiments.
This finding — that cell signal uptake and activation aren't uniform, based on cell layout — could potentially lead to significant medical applications, such as improved chemotherapy delivery.
Michelle Starz-Gaiano, assistant professor of biological sciences, and Bradford Peercy, associate professor of mathematics and statistics, worked with Meyerhoff Graduate FellowLathiena Manning ’15 Ph.D., biological sciences, and Anne Marie Weideman ‘14, mathematics, a current master’s student and alumnus of the NSF-funded interdisciplinary training for Undergraduates in Biological and Mathematical Sciences (UBM) program.
As an animal develops, cells are "fated" to take on different functions, and once fated they go through migration, changes in shape, cell division, and growth to build that organism. Scientists have long sought to fully understand the intricacies of these processes, as errors can have serious consequences for the organism. For example, cell fate malfunctions can play a role in the development of cancers and cleft palate is caused by cells that are fated correctly but don't migrate correctly.
This new research in Nature Communications explores cell fates due to their spatial arrangements, specifically fruit fly ovarian cells that become motile if they get the right chemical signals. Previous science suggested that the closer a cell is to the secreted signal, the higher concentration of signal it would get, and the more likely it would be to become activated and turn into a motile cell. However, this isn't always the case.
Why don't all of the nearby cells respond as theory would predict? To find the answer, the researchers zoomed in for a closer look at the cells and found an important factor not previously examined.
This new paper suggests that the three-dimensional arrangement of cells in relation to one another impacts their response to signals. If you look closely, you can see tiny gaps around the cells, which don't fit completely flush against each other. If there is a gap, a cell is not getting as much activation.
A mathematical model outlined in the paper demonstrates that as that gap fills up with the signal, it shifts from acting as a sink that holds the signal into being a source of signal, which will flow from it. This means that there is a dynamic aspect of cell contours. If you change the landscape of underlying cells, you change activation patterns. Strikingly, the model predicted all of the activation patterns observed in the experiments.
This finding — that cell signal uptake and activation aren't uniform, based on cell layout — could potentially lead to significant medical applications, such as improved chemotherapy delivery.
Tags:
Posted: June 18, 2015, 8:26 AM