UMBC is dedicated to the welfare of both its staff and animals. Once an animal use protocol receives approval, the IACUC diligently oversees all activities involving animals to guarantee adherence to regulations and uphold the utmost standards of animal care and welfare.
Semi Annual Inspections
According to PHS regulations, the Institutional Animal Care and Use Committee (IACUC) must conduct inspections of all animal housing facilities and usage areas at least biannually. These inspections serve as a continuous process to ensure that institutions adhere to relevant policies, guidelines, and laws regarding animal care and use.
The inspections focus on the following areas:
- physical plant condition including functional space, facilities for sanitizing cages, general features of animal housing rooms, composition of floors, walls, and ceilings, lighting, heating, ventilation, and noise control
- laboratory animal facilities including social environment, bedding, water, food sanitation, waste disposal, animal identification, and
- individual laboratories including the physical appearance of the work area, sanitation, use of sterile procedures, storage of anesthetic agents and drugs, record keeping, equipment used for surgery
UMBC Semi Annual Inspections
The UMBC IACUC conducts semi-annual inspections in January and July. Investigators will receive notification of the inspection date and time at least one week beforehand.
PI’s with IACUC approval for using live animals outside the main facility are required to have a lab representative present during inspections. This representative must be well-informed about the animal research protocols and able to access the areas under inspection. Inspections will focus on the rooms indicated in the approved protocol, and it is the Principal Investigator’s duty to verify that this list of rooms is comprehensive and precise.
Tips for Successful Semi-Annual Inspection Results
- Maintain all surgical and post-operative records accurately and ensure they are up to date.
- Store expired drugs separately or dispose of them, contact ESH for guidance on the removal of controlled substances.
- The laboratory and all equipment must be kept clean, organized, and safe for both animals and staff.
- Dispose of expired items like gloves and sutures, or label them for terminal use only when appropriate.
- Label all secondary containers with the necessary information.
- Ensure that food is properly labeled and stored.
Deficiency Areas Identified in the July 2024 Inspection and some general examples of these areas
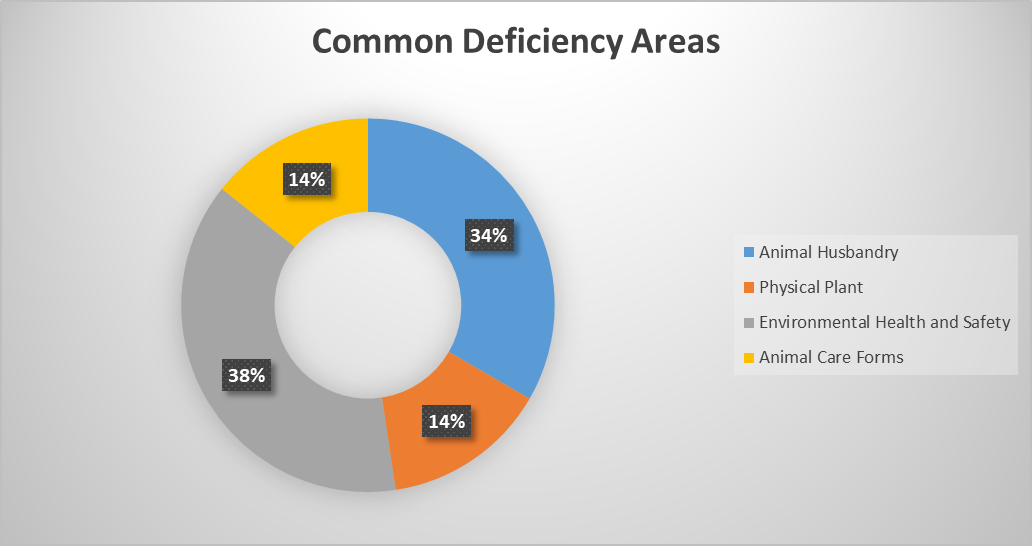
Animal Husbandry 34%
Example: Cage Cards missing Protocol Number.
Recommendations: Ensure Protocol Number is on the card and conduct a retraining with the appropriate staff. Ensure documentation of the training has occurred and is filed.
Example: Single housed cage cards marked with the incorrect protocol number.
Recommendations: Ensure Protocol Number is on the card and conduct a retraining with the appropriate staff. Ensure documentation of the training has occurred and is filed.
Physical Plant 14%
Example: Door frame seal is broken.
Recommendation: Please place a work order to have this repaired and supply a date for when repair will be made.
Environmental Safety and Health 38%
Example: Eye wash station has not been checked since March 2024.
Recommendation: Please complete a retraining with the individual and supply training documentation.
Animal Care Forms 14%
Example: Missing on/off record for lights on 7/11/2024 and 7/12/2024
Recommendation: Conduct a retraining for appropriate staff and upload documentation when complete.
Protocol Specific Review and Site Visit
Specific Review of Animal Use Protocols involves comparing actual activities carried out under an approved protocol to what was written. The review and visit serve as a key method for institutions to ensure that investigators and others involved in animal research adhere to the approved document and maintain related records in compliance (such as surgery records, animal orders, the number of animals used, drugs administered, etc.). The objective of the review and visit is to enhance communication between the IACUC, investigators, and research staff, ensuring the accurate and consistent implementation of animal use as described in the protocols. All protocols are subject to this review and visit at least once during the life of the approved IACUC protocol.
UMBC Protocol Review
The Principal Investigator (PI) will be notified by the IACUC office with an intent notice that includes the review checklist. Approximately one week after the initial notice, a follow-up email will be sent to arrange a visit and address any questions the PI might have. The IACUC Office will carry out protocol audits. Prior to the meeting with the PI and staff, the IACUC Office will examine the animal use protocol and identify specific points for inspection, review, or discussion. The review process may involve comparing procedures executed in the laboratory to those described in the approved protocol. Any discrepancies observed will be reported to the PI.
Discrepancies May Include
- Procedures conducted in the lab are not included in the approved protocol.
- Anesthetics, analgesics, tranquilizers, antibiotics, or other medications utilized in the lab are not recorded in the protocol, differ from those specified, or are not administered according to the protocol.
- Procedures intended to promote animal welfare (e.g., post-operative monitoring) are not being carried out or documented as authorized in the protocol.
- Survival surgeries are not conducted aseptically.
- Euthanasia methods that deviate from the protocol or methods to confirm death (e.g., following CO2 exposure) are not used.
- Laboratory personnel seem to lack the required training to properly conduct procedures outlined in the protocol.
- Necessary documentation for animal care, post-operative care, or other study procedures is incomplete or missing.
- Conditions are unsafe for both humans and animals.
- Expired materials (medications, experimental agents, sutures, sterile supplies, etc.) are being utilized.